Severe tracheo-esophageal fistula induced by stent: repair by membranous tracheoplasty with double esophagus flap
Sancho-Hernández, Rogelio1; Mata-Favela, Nadia Nohemí1; Azuara-Galdeano, Pedro1; Goben-Arredondo, José1
Sancho-Hernández, Rogelio1; Mata-Favela, Nadia Nohemí1; Azuara-Galdeano, Pedro1; Goben-Arredondo, José1
ABSTRACT
KEYWORDS
acquired tracheoesophageal fistula, esophageal flap, stent, tracheoplasty.Introduction
Acquired tracheoesophageal fistula (TAEF) is an abnormal communication between the tracheal airway and the esophageal tract with destruction of the adjacent walls, originating from trauma, malignancy, endotracheal tube and mechanical ventilation-related injury, foreign body aspiration and caustic ingestion.1 Iatrogenic causes induced by an intraesophageal stent are unusual, but they are always severe defects of high mortality and morbidity where because of their severity resection of large tracheal extensions in children is not surgically feasible.
The present report describes the usefulness of a novel surgical procedure with reconstruction of severe stent-induced TAEF by membranous tracheoplasty with double esophageal patch without tracheal resection. There are no national reports describing multidisciplinary treatment of TAEF in children.
Case presentation
An 11-year-old male with a history of caustic ingestion underwent seven unsuccessful esophageal dilatations and endoscopic percutaneous gastrostomy; however, he persisted with stricture, so a 13 and 2 cm metal stent esophageal prosthesis was placed. He was referred to the National Institute of Pediatrics (INP) nine months later due to the difficulty in endoscopic extraction, he presented dysphagia, sialorrhea and productive cough, febrile for six months of evolution, 18 months after the ingestion of caustics.
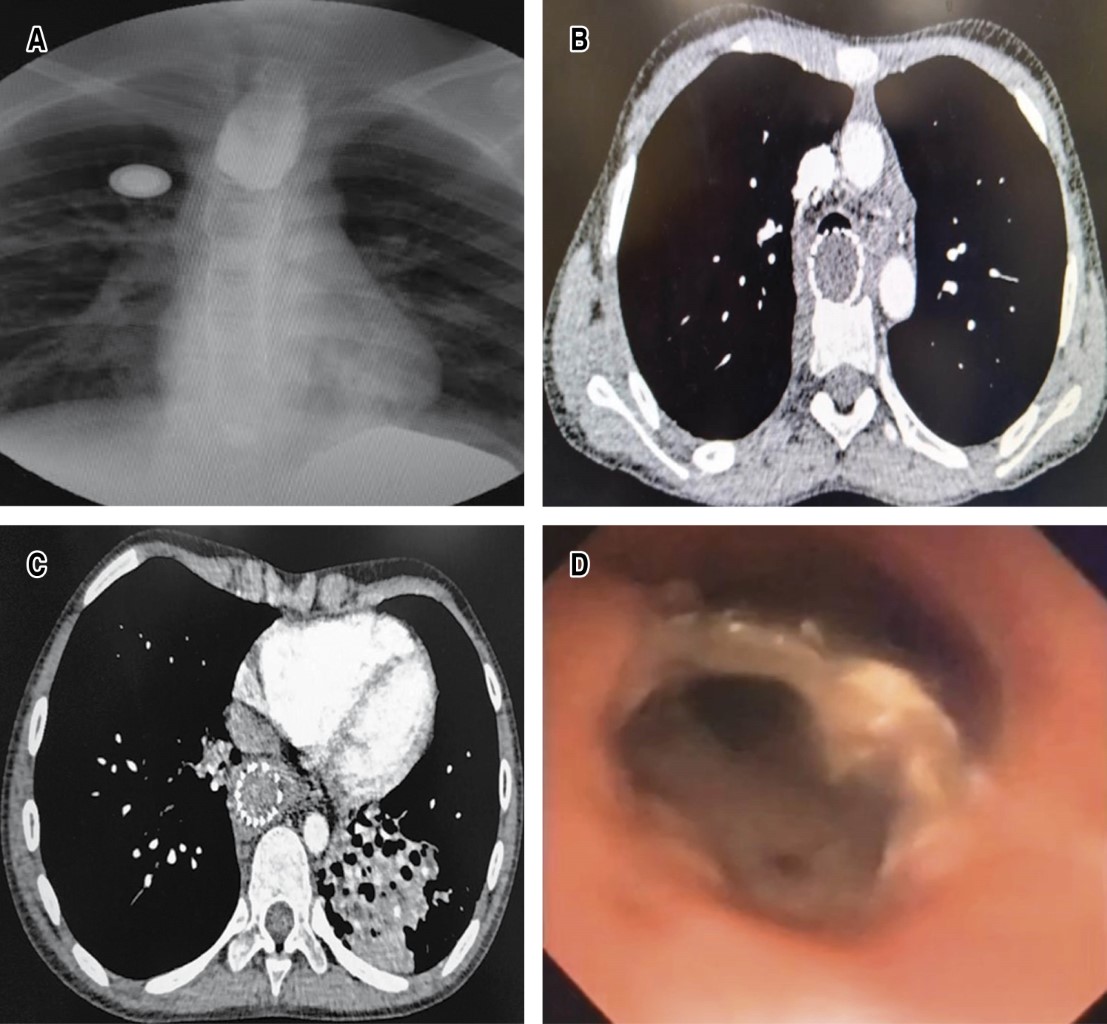
Radiology shows bronchial pattern and presence of intrathoracic radio-opaque esophageal prosthesis, esophagogram shows termination in blind end of the proximal esophagus, gastrogram shows distal esophagus with total stenosis and the distance between both ends of approximately 17 cm; digestive endoscopy shows total esophageal stenosis of the upper third, and the lower esophagus by gastrostomy view with critical and fibrous stenosis > 90% with total esophageal exclusion. The CT scan showed irreversible atelectasis of the left lower lobe due to bronchiectasis; bronchoscopy showed abundant bronchopulmonary suppuration originating in the left bronchus and a foreign body (metallic stent) was observed in the posterior wall of the proximal and middle third of the trachea with radical destruction of the membranous trachea adjacent to the stent, so a diagnosis of TAEF was made. Clinical and nutritional conditions improved with mixed parenteral nutrition and by gastrostomy bronchoscopic drainage of purulent secretions was performed weekly on three occasions and antibiotics were administered (Figure 1A-1D).
Surgical technique
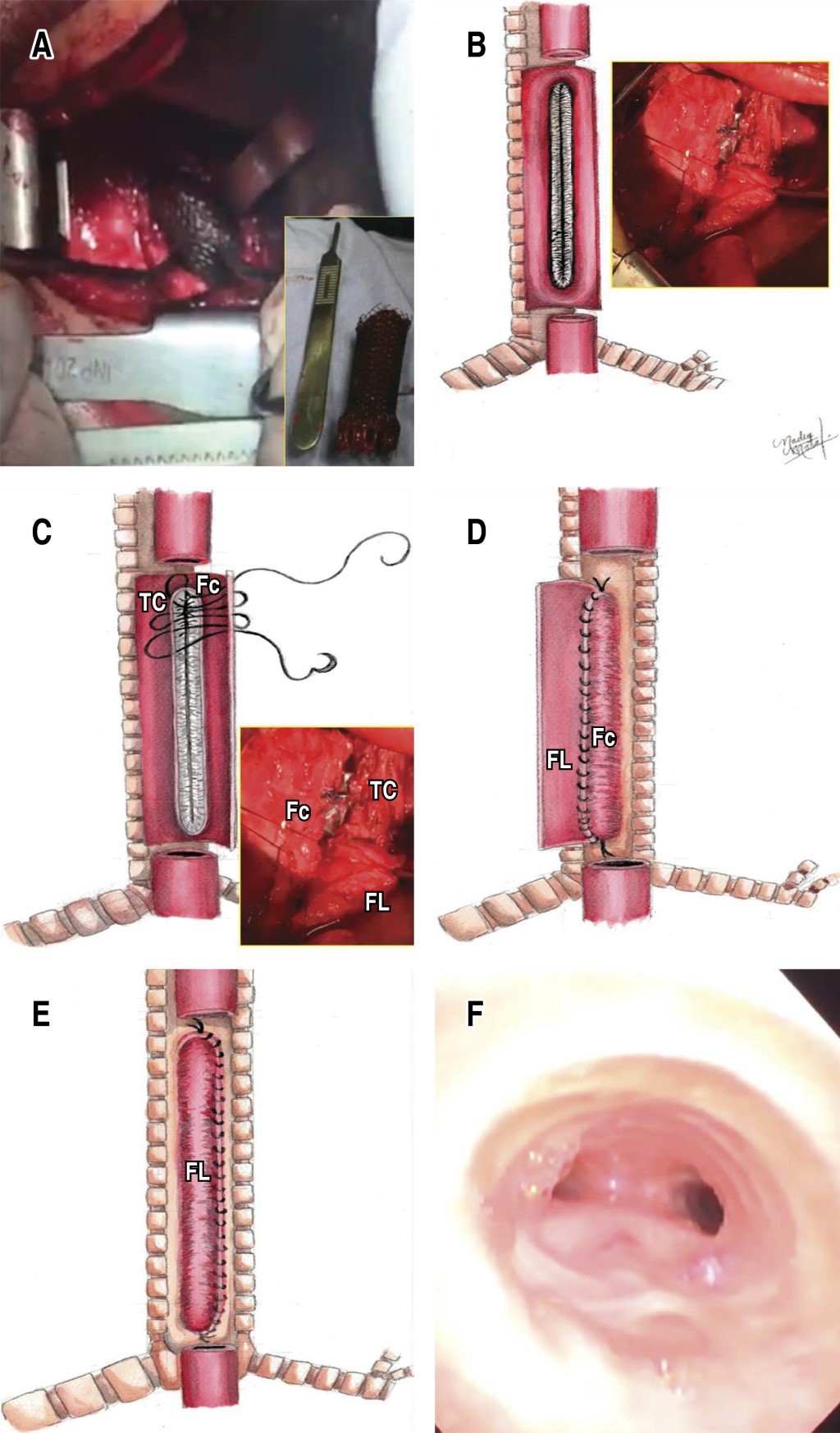
With initial endotracheal intubation guided by bronchoscopy 1 cm below the subglottis, right lateral posterior thoracotomy is performed and esophagus with intense inflammatory reaction is identified, longitudinal esophagotomy is performed, intraesophageal stent is observed, which is removed by fragments with difficulty due to the presence of firm adhesions to the posterior part of the trachea. Once the stent is completely removed, the endotracheal tube is advanced up to 1 cm above the carina, the patient is ventilated with an insufflated balloon and a tracheoesophageal fistula is observed in the membranous portion of the trachea, 10 cm long, the insufflated balloon of the endotracheal cannula allows occluding the air leak and allows distal mechanical ventilation. The edges of the residual esophagus and the excluded membranous trachea are revitalized, the anterior cartilaginous trachea is preserved, esophageal mucosa is removed and the membranous tracheoplasty is performed with two esophageal flaps, A short flap with mucosectomy as the first plane of membranous tracheoplasty anastomosed to the cartilaginous edge of the trachea and a subsequent long esophageal flap covering in a second plane the totality of the neotrachea with simple stitches with 3-0 vicryl. Hemostatic tissue adhesive is placed on the surface of the tracheoplasty and distal esophageal closure is performed in blind end and proximal esophageal derivation as cervical esophagostomy; adequate air tightness of the tracheoplasty is corroborated without air leak and with adequate ventilation by endotracheal cannula in transanastomotic position at 1 cm from the carina (Figure 2A-2F).
In the Critical Care Unit he was kept sedated and with endotracheal intubation for 72 hours and six days after surgery an early revision bronchoscopy was performed where a membranous neotrachea was observed with no data of anastomotic leakage and without complications; a new control bronchoscopy was performed 20 days after surgery where there was again permeability of the neotrachea. Due to persistence of bronchopulmonary suppuration, left lower lobectomy was performed two months after surgery and six months later esophageal substitution method by retrosternal reverse gastric tube, clinical, radiological and endoscopic evolution was favorable at five years of follow-up.
Discussion
TAEF is a severe complication whose etiology in children is divided into malignant acquired causes, which are rare, and benign acquired causes where caustic ingestion, foreign bodies and trauma are the main ones; iatrogenic causes are unusual as in the case presented, which originated from the endoscopic insertion of an intraesophageal stent that is required for the management of refractory esophageal strictures. The prolonged stay of this stent (more than 90 days) caused high pressures on the common wall in the membranous tracheal portion with necrosis that predispose to its development. The incidence of TAEF related to esophageal stents is 4% with a latency of five months after placement.2 The clinical manifestations depend on the size and location of the TAEF, as well as the patient's comorbidities; chronic aspiration pneumopathy, recurrent pneumonias, fever with bronchopulmonary suppuration and chronic malnutrition being the most frequent clinical scenarios, which requires a multidisciplinary evaluation.
Thus, in the preoperative evaluation, esophagogram diagnosis shows TAEF in 70% and in those who cannot swallow or are ventilated, CT scan shows the extension of TAEF or, as in our case, the involvement of adjacent organs and the presence of bronchiectasis; endoscopic evaluation is crucial because it visualizes the location, measurement and characterization of TAEF, facilitates the drainage of purulent material and aspirated gastric contents. Placement of intraesophageal pneumatic balloons and advancing an endotracheal cannula with balloon distal to the fistula to inhibit aspiration of gastric and purulent contents, as well as taking biopsies to orient the etiology, have been reported to be elementary in clinical stability to eliminate the risk of pulmonary sepsis. Suppressing aspiration and associated acute lung damage and allowing an enteral nutrition pathway by means of percutaneous endoscopic gastrostomy and during the transoperative period allowed positioning the endotracheal tube proximal to the fistula to initiate ventilation and subsequently the advancement of the same endotracheal cannula with distal balloon to the TAEF once the stent had been removed and the airway secured, as well as support the reconstruction of the neotrachea and its integrity following membranous tracheoplasty in the immediate postoperative period.2,3
The techniques of esophageal and stricture-involved tracheal ring resection with subsequent primary anastomosis are described for defects greater than 1 cm and up to less than five involved tracheal rings of pediatric tracheal length; in these extensive and complex TAEFs, such combined resections are not surgically feasible;2,4,5 the use of autologous tissue (cartilage, flap or vascularized muscle and/or pericardial flap) or use of other biological covering prosthetic materials have the disadvantage of insufficient blood supply, limited availability, allograft rejection, need to separate and devascularize the surrounding tissue and consequently high recurrence of TAEF, in addition, they may not withstand the high pressures of the compromised airway.6 Endoscopic intervention has been shown to be of pediatric utility for small TAEF < 5 mm with reepithelialization intervention techniques in combination with the application of chemical sealants and/or tissue adhesives that are beyond the scope of this review.7
The use of esophageal and airway metal self-expandable stents, single for distal TAEF or in combination for medial and proximal TAEF placed endoscopically is described in the adult population as temporary bridging measures until a definitive surgical option is reached.2,8 In our patient the stent causing the TAEF could only be removed transoperatively to secure the aeroesophageal pathway, which necessitated definitive surgical correction. Jouraud et al. described the use of the esophageal wall as a biological patch to reconstruct large, inseparable and unsuturable tracheal defects.9 The technique of membranous tracheoplasty reconstruction with double esophageal patch results in a safe and effective surgical method by exhibiting the following qualities: a) it does not require separation of the TAEF avoiding injury to the recurrent laryngeal nerve; b) the esophageal portion of the defect could be used for definitive repair; c) the tracheal portion of the defect could be repaired with the esophageal segment without mucosa to provide stability and rapid recovery of the neotrachea; d) the double patch technique has excellent blood supply and provides support and stability against high airway pressures; e) post-reconstruction recurrence is very low and would allow subsequent success with endoscopic methods; and f) a reconstruction method is needed in the aerodigestive continuity, as in our case, an esophageal substitution method and post-bronchiectasis lung resection.6,10
Conclusions
Intraesophageal stent-induced TAEF is a severe defect with high mortality and morbidity, multidisciplinary management and bronchoscopic evaluation of the pediatric aerodigestive model are crucial in clinical stability to eliminate pulmonary sepsis, suppress aspiration and associated acute lung damage, and allow a nutritional pathway. The use of adjacent esophageal wall with membranous tracheoplasty with double esophageal patch is a safe and effective method in aerodigestive reconstruction and is an opportune option where due to its severity resection of large tracheal extensions in children is not surgically feasible and endoscopic treatment is not a conservative option.
AFILIACIONES
1Instituto Nacional de Pediatría. Mexico City, Mexico.Acknowledgements: Intensive Care Unit of the National Institute of Pediatrics, Mexico City, Mexico.
Conflict of interests: the authors declare that they have no conflict of interests.
REFERENCES