Tuberculosis and BCG vaccine: role of NK cells in the immune response
Rojas-Valles, Edwin Uriel1,2; Antonio-Pablo, Roberto Carlos3; Herrera-Barrios, María Teresa2
Rojas-Valles, Edwin Uriel1,2; Antonio-Pablo, Roberto Carlos3; Herrera-Barrios, María Teresa2
ABSTRACT
The innate immune is the first line of defense of the immune system and is characterized by the rapid response against infectious agents through the recognition of molecular patterns. Within the cells of innate immunity are natural killer cells, which show cytotoxic activity against infected or transformed cells. They have activation, inhibition and natural cytotoxicity receptors that allow their activation, causing the release of perforins, granzymes B and granulysins contained in their cytoplasmic granules which participate in the elimination of target cells. Furthermore, natural killer are a source of cytokines such as IFN-γ, TNF-α, IL-10, IL-2 and GM-CSF. They are important source of IFN-γ which promotes the activation of bactericidal mechanisms in macrophages in defense against intracellular pathogens such as Mycobacterium tuberculosis, which causes tuberculosis. Tuberculosis is an infectious disease that represents a global health problem, and the only preventive measure is the BCG vaccine, which is generally applied at birth. Natural killer cells have been reported to participate in immunity against tuberculosis, as well as in the protection conferred by BCG. The objective of this review is to highlight the most important findings on the role of natural killer cells in tuberculosis and in response to BCG vaccination in humans and animals, which may open a broader panorama to propose new preventive measures or therapies against tuberculosis, infections or cancer.KEYWORDS
NK cells, tuberculosis, BCG vaccine, innate immunity, trained immunity.Abbreviations:
Introduction
All living organisms have an immune system that protects them from pathogens that can cause disease. This system involves various cell lineages with specific functions in host defense. NK cells are involved in defense in tuberculosis and it is important to know their role in infection and as part of the response to a vaccine such as BCG.
Tuberculosis
Tuberculosis is an infectious disease caused by M. tuberculosis, posing a global public health problem. It is the second leading cause of death from a single infectious agent after COVID-19; it has been estimated that a quarter of the world's population is infected.1 In 2022, the WHO reported 10.3 million new cases and 1.3 million deaths from tuberculosis.1
It is transmitted by the airborne route, when a person with PTB expels the mycobacteria through coughing or sneezing; these remain suspended in the environment and are inhaled by other people and the fate of the infection will be determined by the immunity of the person and the virulence of the Mycobacterium. When M. tuberculosis reaches the pulmonary alveoli it is phagocytized mainly by resident macrophages, initiating cytokine production and migration of monocytes to the site of infection, where they will differentiate into macrophages. However, M. tuberculosis is also phagocytosed by DCs, which migrate to thoracic lymph nodes to present mycobacterial antigens to naïve T-cells, leading to the proliferation and differentiation of antigen-specific CD4+ or CD8+ T-cells. These cells migrate to the site of infection, surrounding infected and uninfected cells in the lung, forming part of the multicellular structure called granuloma. It has been speculated that the granuloma prevents and contains the spread of M. tuberculosis to extrapulmonary sites, but it has also been considered a niche that mycobacteria exploit to persist in the host.2
Most infected individuals (90-95%) control the infection and have LTBI, characterized by no signs or symptoms of TB and no contagiousness, but produce interferon gamma (IFN-γ) in response to mycobacterial Ag (IGRA positive). However, 5-10% develop active TBP within two years, associated with factors that reduce the immune response such as: malnutrition, HIV infection, compromised immune system, smoking, alcoholism and diabetes mellitus.3-6
Innate immunity
In the lung, cells of innate immunity represent the first line of defense when M. tuberculosis reaches the pulmonary alveoli. Macrophages, DCs, neutrophils and NK cells interact with the Mycobacterium to try to control infection and prevent disease.7 However, M. tuberculosis can infect cells and replicate to persist in the host using its evasion mechanisms, such as: 1) altering phagosome-lysosome biogenesis, 2) producing components (PtpA, 1-TbAd and MarP) to neutralize and tolerate the acidic environment of the phagosome, 3) causing phagosomal membrane rupture to escape to the cytosol and gain access to nutrients using the ESX-1 secretion system, 4) causing plasma membrane rupture to infect nearby cells, and 5) inhibiting inflammasome activation, pyroptosis and autophagy.8
M. tuberculosis recognition
Cells of innate immunity recognize pathogen-associated molecular patterns of M. tuberculosis through their PRRs, such as: C-type lectins (mannoses receptors, DC-SIGN, dectin-1, dectin-2 and Mincle); NOD-type receptors; complement receptors (CR3); collectins (surfactant proteins A and D, mannose-bound lectin); scavenger receptors (MARCO, SR-A1, CD36, SR-B1); Fc receptors (FcgR); glycophosphatidyl-inositol-anchored membrane receptors (CD14); and Toll-like receptors (TLR-2, TLR-4 and TLR-9).9
Particularly, different PRRs of innate immunity cells that recognize M. tuberculosis Ag have been described (Table 1A), allowing phagocytosis and/or host defense through cytokine production (IL-1ß, IL-6, TNF-α), autophagy and inflammasome activation.9
Mycobacterial antigens modifying the immune response
M. tuberculosis has components that promote or inhibit host defense mechanisms such as phagocytosis, autophagy, apoptosis, and inflammasome (Table 1B).10
BCG vaccine
In 1908 Léon Charles Albert Calmette and Jean-Marie Camille Guérin initiated the attenuation of M. bovis isolated in 1902 from a tuberculous cow and generated the live-attenuated vaccine against tuberculosis.11,12 They made 231 serial passages of M. bovis over 13 years, until in 1921 they obtained the M. bovis BCG (Bacillus Calmette and Guérin) strain, which conferred protection against tuberculosis in guinea pigs.11-13
In 1921, the BCG vaccine was administered for the first time in a child exposed to tuberculosis bacilli, demonstrating that after two years of constant exposure he did not present lesions and signs of tuberculosis.11,14 Therefore, from 1924 onwards, this vaccine was distributed to 20 countries for its application by WHO recommendation.12,15
Genomic studies have shown that the BCG strain lacks the difference region-1 (RD-1) present in M. tuberculosis and M. bovis.13 In this region are genes encoding proteins involved in the secretion system (Rv3876, Rv3877 and Rv3878) and virulence factors important in the pathogenesis of the disease (Rv3871, PE35, PPE68, Rv2879c, CFP-10 and ESAT-6).11,13,16-20
Innate immunity to BCG
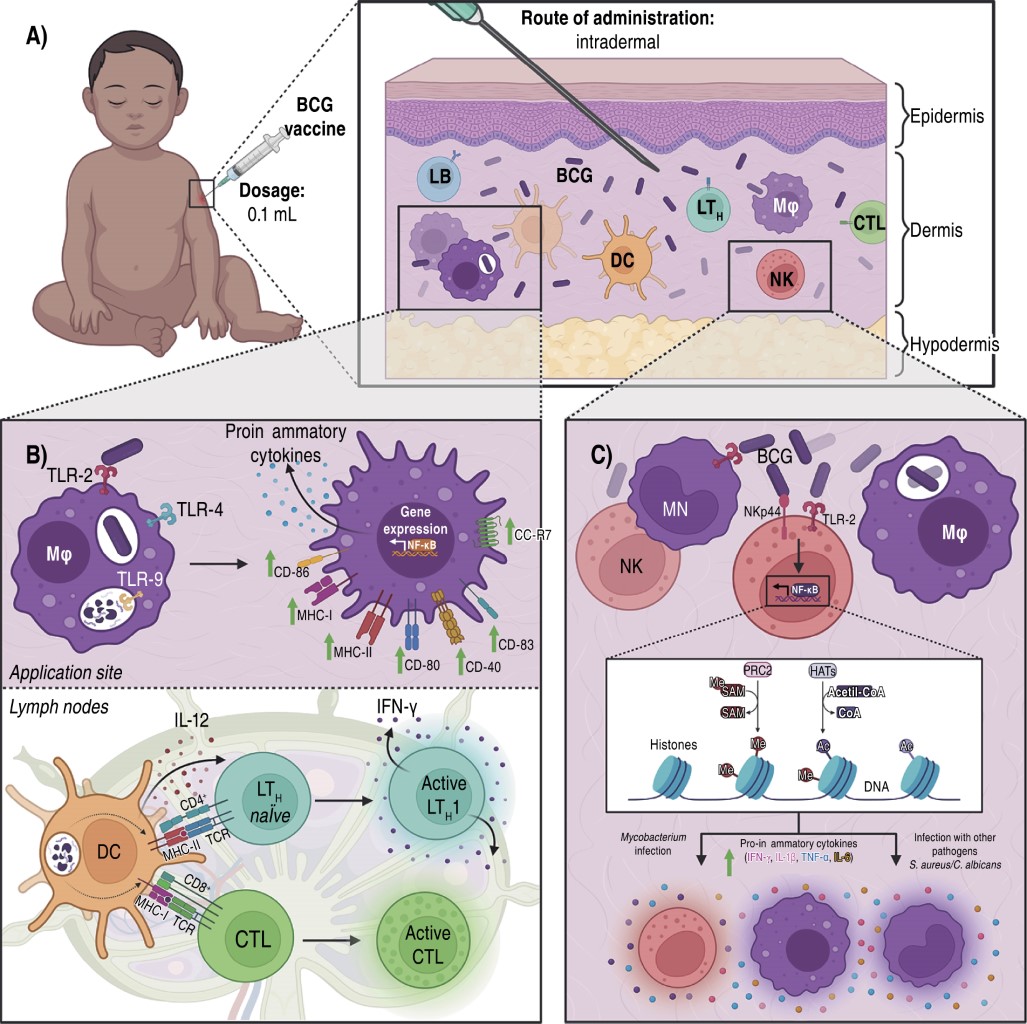
BCG vaccine is applied intradermally causing a local immune reaction (Figure 1A), which is initiated by the recognition of the bacilli by macrophages and DCs, which increase the expression of MHC class I and class II molecules, co-stimulatory molecules (CD40, CD80, CD83 and CD86) and the chemokine receptor 7 (CCR7); favoring migration to lymph nodes and the processing and presentation of Ag to T-cells.12,21 On the other hand, macrophages recognize, phagocytize and degrade BCG by activating different PRRs such as TLR-2, TLR-4 and TLR-9; which stimulates the expression and secretion of proinflammatory cytokines (IL-6, IL-12, TNF-α and MCP-1), favoring a TH1 response involving CD4+ T-cells and, in addition, the activation of CD8+ T-cells.12 This response is characterized by the production of IFN-γ, a crucial cytokine in the protection against intracellular pathogens such as M. tuberculosis, because it activates the bactericidal mechanisms of macrophages (Figure 1B).12
This vaccine also induces non-specific trained immunity (Figure 1C), based on epigenetic reprogramming in monocytes, macrophages and NK cells. So the cells respond rapidly and strongly to secondary Mycobacterium infections and even to different pathogens.12,15 This epigenetic reprogramming takes place in histones by methylation, acetylation, deamination and proline isomerization at the promoter sites of genes coding for proinflammatory cytokines.12 Thus NK cells produce proinflammatory cytokines (IFN-γ, IL-1β, IL-6 and TNF-α) in response to BCG-related and non-BCG-related pathogens after two to 12 post-vaccination weeks.15
While it has been suggested that NK cells are crucial in cross-protection against other BCG-induced pathogens, their importance in response to BCG vaccination has not been described. Thus, we will describe recent reports below.
NK cells
They belong to the lymphoid lineage and have cytotoxic activity against infected (virus, bacteria or parasites) or tumor cells.22,23 They were first described in the 1970's, and their importance in innate immunity is currently recognized.24 They are classified within the innate lymphoid cells, although under certain circumstances they show adaptive and memory characteristics. They are part of the first line of defense involved in the recognition and elimination of infected or transformed cells; in addition, they produce IFN-γ, IL-6, TNF-α and chemokines such as MIP-1α, MIP-1ß and IL-8.25-27 They are mainly located in the blood (5-20% of circulating lymphocytes in humans) and lymph nodes, as well as in the skin, intestine, liver and lungs.22,28
Origin and morphology
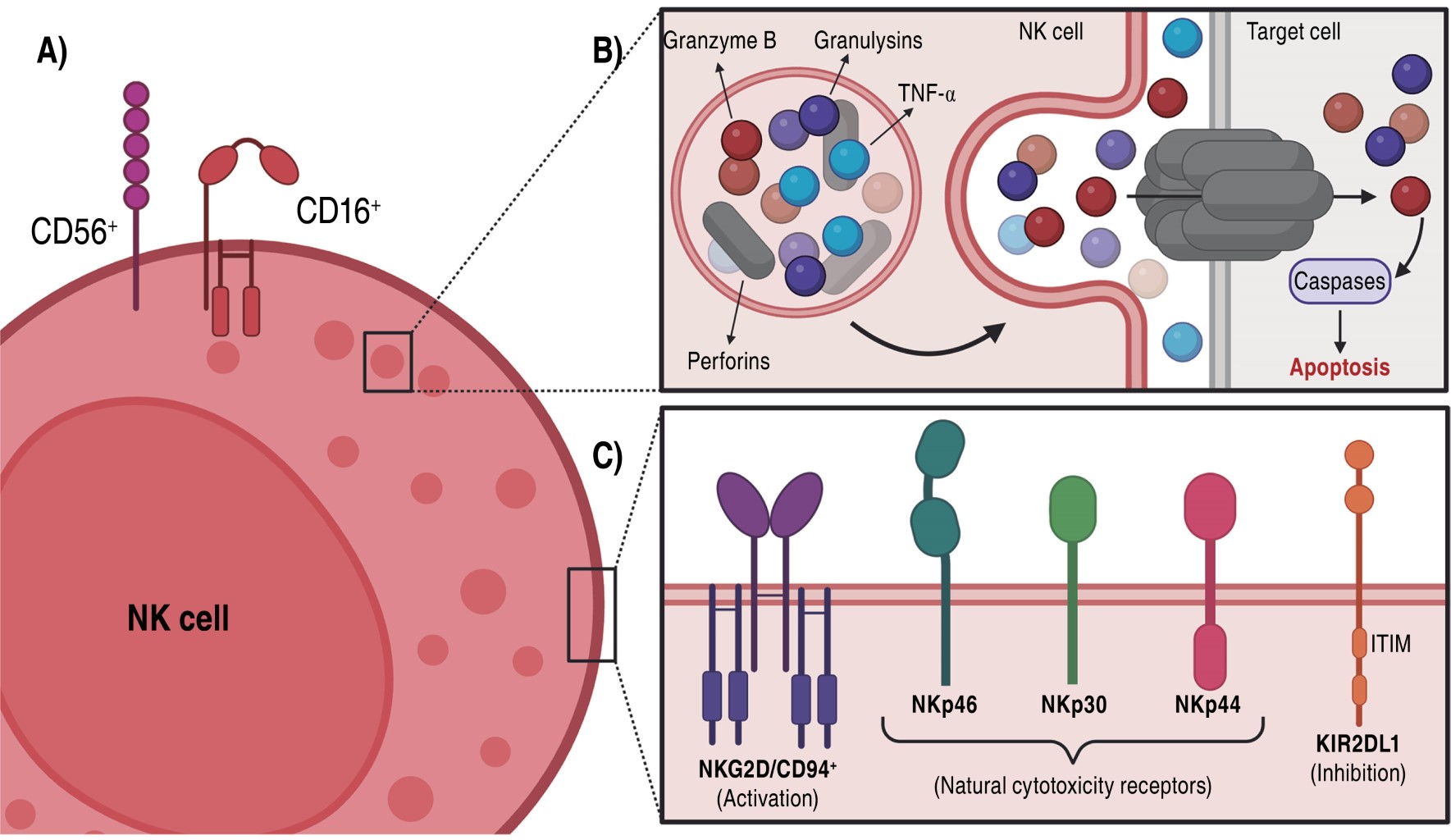
They originate in the bone marrow from a pluripotential hematopoietic stem cell (CD34+), which will give rise to a lymphoid progenitor. The process of differentiation and maturation begins when the lymphoid progenitor derives into a T/NK biopotential that mediated by IL-12, IL-7 and IL-15 and the transcription factors eomesodermin (EOMES), E4BP4, ld2, BLIMP and T-bet will guide the development into an immature NK cell to a mature NK cell. These cells express specific surface markers (CD56+ and CD16+) (Figure 2A) that allow them to be differentiated from T (CD3+) and B (CD19+) cells. Two subpopulations have been described according to their maturation: CD56brightCD16- (90% in peripheral blood) and CD56dimCD16+, the latter showing greater cytotoxicity.29-32
NK cells are granular cells (10 to 15 mm) with little rough endoplasmic reticulum, mitochondria and free ribosomes. They are characterized by cytoplasmic granules containing cytolytic enzymes such as perforins, granzyme B, granulysins, proteoglycans and TNF-α (Figure 2B).30,33
NK cell receptors
These cells on the membrane have receptors that allow them to interact with other cells and thus identify infected or tumor cells (Figure 2C). They possess: a) C lectin-like receptors: NKG2D/CD94, which recognize human leukocyte Ag E5 (HLA-E5); b) KIR (Killer Inhibitory Receptor) activation and inhibition receptors that function by detecting the increase or decrease of HLA-I6 molecules; c) natural cytotoxicity receptors, specific to NK cells that include activating receptors such as NKp30, NKp44 and NKp46; and d) the CD16 receptor (IgG low affinity Fc receptor, FcgIII) that participates in recognition of opsonized cells.22,31 Some of the activating and inhibitory receptors, as well as their ligands, are described in Table 2.22,34-38
Functions
They are innate effector cells that participate in the response against pathogens, infected or tumor cells, in addition to favoring an adaptive immune response.26,31,39 Their cytotoxic activation occurs upon contact with infected or tumor cells that lack MHC-I, or MHC-I is altered. They can also be activated with IL-2, favoring their action against tumors. This cytotoxic activity can be inhibited by recognition of MHC-I in target cells, which prevents cell lysis.35,40,41 Another of their functions is the production of cytokines (IFN-γ, IL-10, TNF-α, GM-CSF) after their activation and subsequent stimulation with IL-12.35
Due to the cytokine profile they produce they can be classified into: NK1, secreting IFN-γ, IL-10, TNF-α and GM-CSF; and NK2, secreting IL-5, IL-13, TNF-α and GM-CSF. IFN-γ production is the most important impact on the host immune system since it promotes the bactericidal mechanisms of macrophages, such as phagocytosis and IL-12 production, promoting IFN-γ production and the development of the TH1 response; it also modulates the immune response of T cells and DC.26,31,35
Role of NK cells in tuberculosis
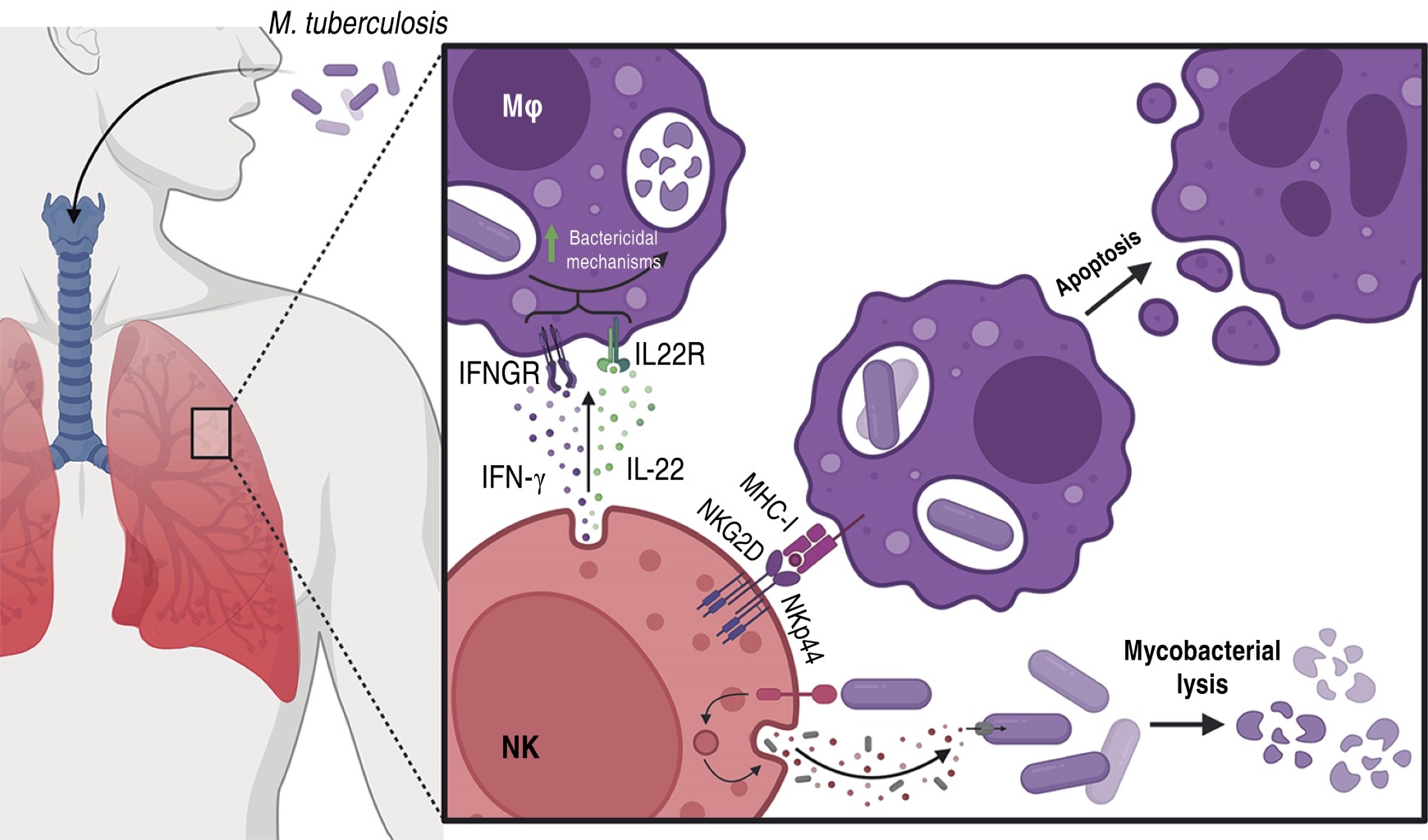
NK cells have cytolytic capacity acting at the initial stage of infection without MHC restriction. It has been described that NK cell protection against virus, bacterial and parasitic infections is based on cell recognition and cytolytic effect, production of IFN-γ, IL-12, IL-22, TNF-α and GM-CSF, and secretion of cytolytic proteins (perforin, granzymes and granulysins). They carry out lysis of human monocytes and macrophages infected with M. tuberculosis through interaction with their natural membrane cytotoxicity receptors NKp36 and NKG2D.42 There are components of M. tuberculosis that are recognized by receptors present on NK cells (Figure 3), such is the case of mycolyl-arabinogalactan-peptidoglycan (mAPG), mycolic acids (MA) and arabinogalactan (AG) that are ligands of the NKp44 receptor; while peptidoglycan binds only to TLR-2. During TBP and meningeal tuberculosis in humans, peripheral blood NK cells are activated by showing increased CD69+ activation marker, but show a significant reduction of NKp36 and NKG2D receptors in LTBI and PTB compared to healthy individuals, which may affect the recognition and control of M. tuberculosis (Figure 3).43 These cells can inhibit the growth of M. tuberculosis H37Rv in infected human macrophages through the production of IL-22, which promotes phagolysosomal fusion, or by degranulation of their cytolytic proteins (perforins, granzymes and granulysins) (Figure 3), through the MAPS kinase pathway (ERK, JNK, p38) mediated by NKG2D receptors.44,45
NK cell response in BCG vaccination
The NK cell response stimulated by BCG vaccination is considered to be of great interest, as it may elicit cross-immunity against different types of infections and various cancers. This has led to research using animal models and, in addition, the response of these cells has been evaluated in humans following BCG vaccination.
Animal model response
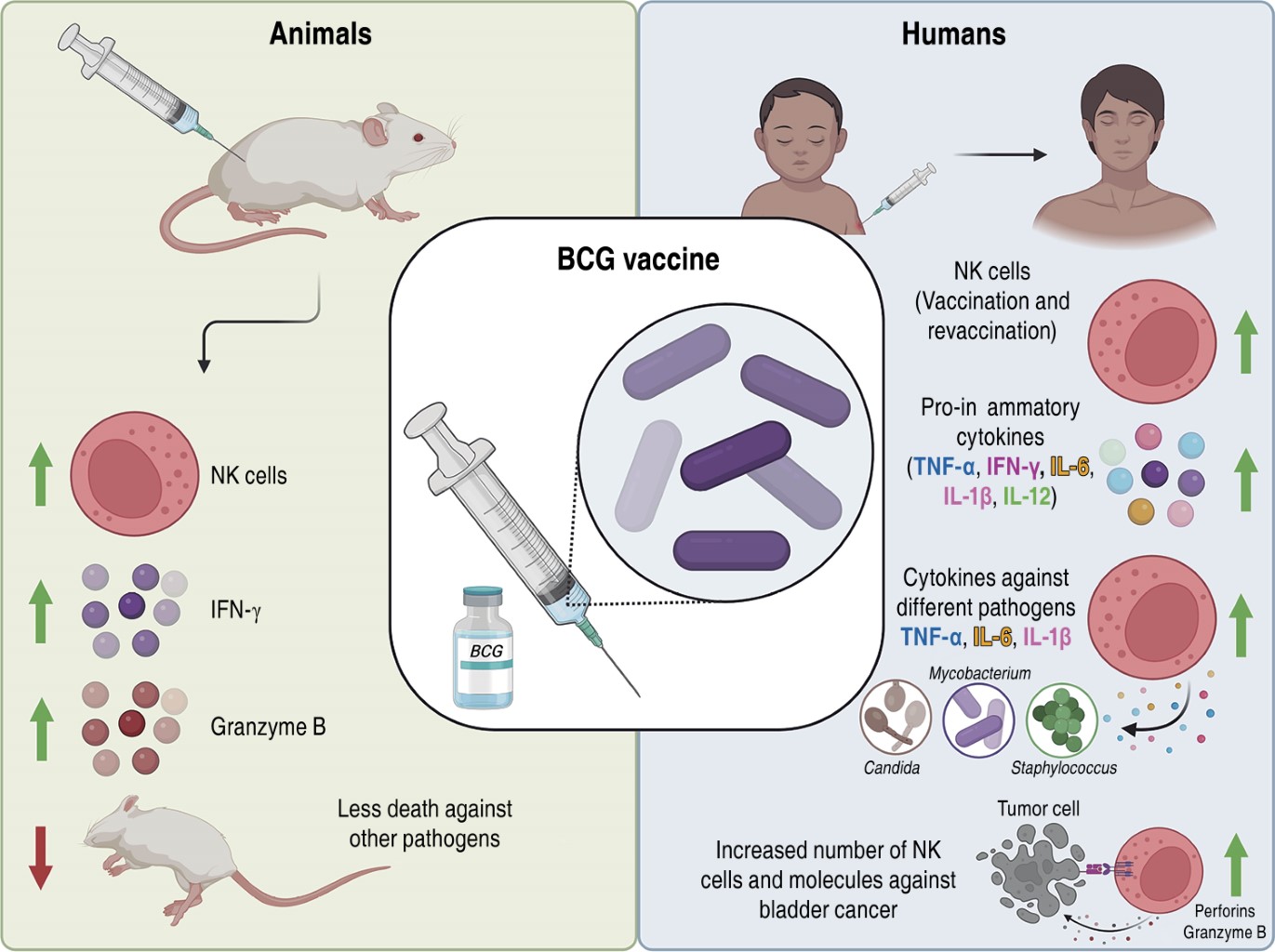
After immunization of mice with BCG, there is an activation of NK cells as producers of IFN-γ, which favors the bactericidal capacity of macrophages.46 In addition, post-vaccination there is migration of neutrophils to the site of infection to subsequently produce IL-12 and TNF-α that will serve to stimulate the migration, survival and action of NK cells as a source of IFN-γ. This favors the increase of NK cells at the site of infection and in the lymph nodes, reaching the maximum peak of cells in the lung and spleen at seven days, together with IFN-γ production at five days.47,48 BCG-generated immunity is partly due to NK cells, as they promote TH1 cell activation. Some of the characteristics of BCG-treated NK cells are increased granzyme B expression, IFN-γ, CD107 and CD11b levels, and cytotoxicity against mouse melanoma-like tumor cells (B16F10).46 Other studies have highlighted the importance of NK cells in protection, as they have reported that SCID (severe combined immunodeficient, lacking T and B-cells) and NSG (NOD/SCID/IL2RG, lacking T, B and NK cells) mice were vaccinated with BCG or saline (control) and subsequently challenged with Candida albicans. They observed that 100% of vaccinated SCID mice survived compared to 30% of the control group; while NSG mice gradually died. This result supports the importance of the presence of NK cells in the protection conferred by BCG to other infections.49
Human response
As already mentioned, these cells are an important source of cytokines, since cord blood from newborns exposed to BCG favors the production of IFN-γ, IL-10, IL-12, IL-13 and IL-15; demonstrating that only NK cells are the source of IFN-γ, while monocytes produce IL-10 and IL-12.50 On the other hand, it has been demonstrated that NK cells purified from peripheral blood of healthy volunteers cultured with M. bovis BCG, show an activated state (increased CD69+CD25+), and are able to produce elevated levels of IFN-γ and TNF-α, in addition to showing cytotoxic activity and capacity to destroy immature DCs through a mechanism involving TLR-2.51
In South Africa, a study in the BCG-vaccinated newborn population showed increased IFN-γ-secreting NK cells at five and nine weeks post-vaccination compared to the unvaccinated.52 In addition, vaccinated children showed increased secretion of IL-12, IFN-γ, IL-6, IL-1β and TNF-α in plasma, suggesting protection against cytokine-mediated mycobacterial infections.52 Until a few years ago, it was believed that innate cells had no memory; however, revaccination with BCG in adults increased the number of CD56brightCD16- and CD56dimCD16+ NK cells, which persisted in peripheral blood for more than one year.53
Trained immunity has been studied in CD56+ NK cells from healthy volunteers isolated from blood before vaccination, two weeks and three months post BCG vaccination. CD56+ NK cells stimulated with sonicated M. tuberculosis H37Rv, C. albicans and inactivated Staphylococcus aureus were able to produce elevated amounts of proinflammatory cytokines (IL-1β, IL-6, TNF-α) compared to the basal condition. This demonstrated that NK cells have immunological memory by having an increased proinflammatory response and recognition capacity to other mycobacteria and other non-BCG microorganisms.49 Other studies have reported that peripheral blood mononuclear cells from healthy volunteers stimulated with BCG induce activation and proliferation of CD56bright NK cells.54 In addition, co-culture of CD56bright NK cells with BCG showed elevated levels of perforins, granzyme B and IFN-γ, as well as efficient degranulation against bladder cancer cells.54 BCG-treated bladder cancer patients have shown increased CD56bright NK cells, suggesting that BCG-induced NK cell activation may be an important component of the immune response against bladder cancer.54
Conclusions
Tuberculosis is a disease that affects the world's population and the BCG vaccine is the preventive measure. However, this vaccine has been shown to favor immunity trained on innate cells such as NK cells. Considering together the findings we have described above (Figure 4), BCG-activated NK cells play an important role in the development of trained immunity against infections by other mycobacteria or unrelated pathogens and as antitumor therapy against bladder cancer. Thus, new lines of research can be generated to propose new drugs or therapies against various infections or cancers by mediating NK cell activation.
AFILIACIONES
1Universidad Nacional Autónoma de México. Mexico City, Mexico. 2Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas. Mexico City, Mexico. 3Universidad Autónoma Metropolitana, Unidad Xochimilco. Mexico City, Mexico.Acknowledgments: to Edwin Uriel Rojas-Valles and Roberto Carlos Antonio-Pablo for their valuable contributions in the preparation of the manuscript.
Conflict of interests: the authors declare that they have no conflict of interests.
REFERENCES
Rojas-Valles EU. Evaluación del crecimiento intracelular y la producción de citocinas pro-inflamatorias por los leucocitos de sangre periférica frente a la infección con Mycobacterium bovis BCGΔBCG1419c y Mycobacterium bovis BCG?BCG1419c::Rv1354c, micobacterias candidatas a vacuna contra la tuberculosis pulmonar (tesis). 2023:1-41. Accesible en: http://132.248.9.195/ptd2023/febrero/0835821/Index.html
García DA, Pérez P, García L, Cid-Arregui A, Aristizabal F. Expresión génica de ligandos mica, micb y ulbp (1-6) del receptor NKG2D de células natural killer y metaloproteinasas adam10, adam17 y mmp14 en líneas celulares de cáncer de cervical. Rev Colomb Biotecnol. 2019;21(1):29-38. doi: 10.15446/rev.colomb.biote.v21n1.79730.
|
Table 1: Receptors of innate immunity cells and effect of M. tuberculosis components. |
||
|
A) Receptors of innate immunity cells and their mycobacterial ligands. |
||
|
Cell |
Receptor |
M. tuberculosis antigens |
|
Macrophage |
TLRs MR CD91, calreticulin |
LM, LAM, ManLAM, PIM, Hsp60/65, DNA y RNA LAM ManLAM, MBL |
|
DC |
TLRs DC-SIGN |
LM, LAM, ManLAM, PIM, Hsp60/65, DNA, RNA ManLAM |
|
NK |
NKp44 NKp46 NKp30 NKG2D TLR-2 |
MA, mAPG, AG M. bovis BCG
M. bovis BCG PG |
|
B) Effect of M. tuberculosis antigens on defense mechanisms. |
||
|
Defense mechanism |
Favored antigen |
Inhibited antigen |
|
Phagocytosis |
PPE57 |
PIMs, ManLAM, PKG, PtpA, EIS |
|
Autophagy |
ESAT-6, c-di-AMP |
EIS, SapM, LrpG, PDIM |
|
Apoptosis |
LpqH, PE _PGRS3 3, ESAT- 6, OppD, PstS1, Rv0183, Rv0901, PE9/PE10, Mce4A |
PtpA, NuoG, PknE, SecA2, SodA, SigH, MPT64, Rv3354 |
|
Inflammasome |
EsxA, Mtb DNA |
Zmp1 |
|
AG = arabinogalactan. c-di-AMP = cyclic di-adenosine monophosphate. DC = dendritic cells. EIS = enhanced intracellular survival. ESAT-6 = early secreted antigenic target of 6 kDa. LAM = lipoarabinomannan. LM = lipomannan. LrpG = leucine-responsive regulatory protein G. MA = mycolic acids. ManLAM = monosylated lipoarabinomannan. mAPG = mycolyl-arabinogalactan-peptidoglycan. MBL = mannose-binding lectin. Mce4A = mammalian cell entry complex 4A. MPT64 = M. tuberculosis Protein 64. NK = natural killers. NuoG = subunit of NADH dehydrogenase type I. OppD = oligopeptide permease D. PDIM = phthiocerol dimycocerosates. PE9/PE10 = protein proline-glutamate 9/10. PG = peptidoglycan. PIM = phosphatidyl inositol mannoside. PKG = protein kinase G. PknE = protein kinase E. PPE57 = protein proline-proline-glutamate 57. PstS1 = phosphate-specific transport substrate binding protein-1. PtpA = protein tyrosine phosphatase. MR = mannose receptor. SapM = secretory acid phosphatase. SigH = Sigma factor H. TLR = toll-like receptors. Zmp1 = zinc metalloprotease. |
||
|
Table 2: Receptors for activation and inhibition of NK cells. |
||
|
Activation receptors |
Linking |
Reference |
|
NKG2C NKG2D NKG2E KIR2-DS1 KIR2-DS2 KIR3-D NKp30 NKp44 NKp46 NKp80 CD16 CD94 DNAM-1 |
HLA-E MIC (a and b) and ULBP (1-6) HLA-E HLA-C2 (lysine in position 80) HLA-C2 with viral peptide HLA-F B7-H6, HCMV-pp65, BAG6, heparan sulfate MLL5, viral hemagglutinin, PCNA, PDGF-DD Complement factor P, viral hemagglutinin, heparan sulfate AICL IgG antibody complex section HLA-E CD155 and CD112 |
34, 35 34-36 34, 35 34, 37 34, 37 34, 35 22, 34 22, 34 22, 34 38 34, 35 34, 35 34, 38 |
|
Inhibitory receptors |
||
|
KIR2DL1 KIR2DL2/3 KIR3DL1 KIR3DL2 NKG2A NKRP1A KLRG1 PD1 |
HLA-C2 (lysine in position 80) HLA-C1 (asparagine in position 80) and HLA-C2 (lysine in position 80) HLA-A, HLA-Bw4 HLA-Aw3, HLA-Aw11 Inhibitory peptide in HLA-E LLT1 Cadherins PDL1-L2 |
34 34 34 34 34 34 34 38 |
|
AICL = activating inducing ligand. BAG6 = scythe protein. HCMV = human cytomegalovirus. HLA = human leukocyte antigen. LLT1 = lectin-like transcript. MIC = class I chain-related protein. MLL5 = mixed lineage leukemia 5. PCNA= proliferating cell nuclear antigen. PDGF-DD = platelet-derived growth factor-DD. PDL1 = programmed death-ligand 1. ULBP= UL16-binding proteins. |
||


